Genomic instability in cancer
I. Genomic instability during early stages of cancer development
Cancer development requires the acquirement of several capabilities that allow cells to survive, proliferate and spread without being subjected to cellular regulatory barriers. Genomic instability, a hallmark of most cancer cells, is a crucial mechanism which enables the acquirement of new characteristics required for tumorigenisity. During early stages of cancer development, genome instability is the result of global changes in the DNA replication program, transcription profiles and epigenetic marks, which all lead to perturbed DNA replication and DNA damage.
We study the signaling pathways and key factors involve in the altered replication dynamics during early stages of cancer, focusing on oncogenes and tumor suppressor genes which regulate the replication process. We also study environmental factors which can affect the replication dynamics and support cancer development. Altogether, we try to elucidate how changes in the genomic landscape of replication sensitivity regulate the fate of normal cells enforced by cancer genes to proliferate and develop into malignant tumors.
Our research is aimed to unfold the molecular basis underling genome instability in cancer and open new potential prevention and therapeutic approaches.
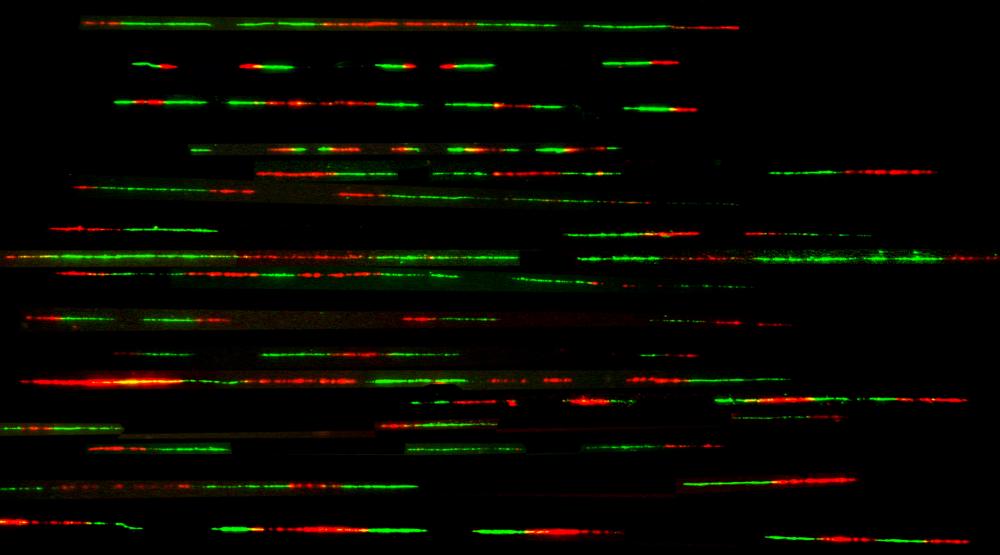
Analysis of DNA replication dynamics by DNA combing
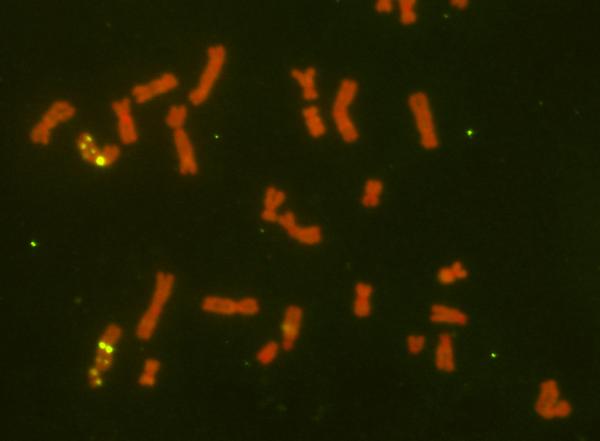
II. Genomic instability at human common fragile sites
Maintenance of genome integrity is of great importance since genomic instability is one of the early events driving cancer progression. We focus on specific human genomic loci defined as common fragile sites (CFSs), which are an intrinsic part of the normal human genome and are preferentially unstable under conditions of DNA replication stress. These loci are preferential sites of frequent chromosomal breaks and rearrangements in all stages of cancer development. We study different aspects of the complex molecular basis underlying the instability at these sites by analyzing the replication dynamics and DNA damage along them. As genomic instability at CFSs alters the expression of important cancer genes in different cancer types, elucidate CFSs molecular characteristics can shed light on the initial events driving tumorigenicity.